
SL Paper 1
The reaction below represents the Haber process for the industrial production of ammonia.
\[\begin{array}{*{20}{l}} {{{\text{N}}_2}{\text{(g)}} + 3{{\text{H}}_2}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_3}({\text{g)}}}&{\Delta {H^\Theta } = - 92{\text{ kJ}}} \end{array}\]
The optimum conditions of temperature and pressure are chosen as a compromise between those that favour a high yield of ammonia and those that favour a fast rate of production. Economic considerations are also important.
Which statement is correct?
A. A higher temperature would ensure higher yield and a faster rate.
B. A lower pressure would ensure a higher yield at a lower cost.
C. A lower temperature would ensure a higher yield and a faster rate.
D. A higher pressure would ensure a higher yield at a higher cost.
Markscheme
D
Examiners report
Which experimental methods could be used to observe the progress of the following reaction?
Cr2O72-(aq) + 6I-(aq) + 14H+(aq) → 2Cr3+(aq) + 3I2(aq) + 7H2O(l)
I. Change in colour
II. Change in mass
III. Change in electrical conductivity
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Which statements describe the action of a catalyst?
I. It does not alter the \(\Delta H\) for a reaction.
II. It increases the \({E_{\text{a}}}\) for the reaction.
III. It alters the mechanism (pathway) of a reaction.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Consider the reaction between magnesium and hydrochloric acid. Which factors will affect the reaction rate?
I. The collision frequency of the reactant particles
II. The number of reactant particles with \(E \geqslant {E_{\text{a}}}\)
III. The number of reactant particles that collide with the appropriate geometry
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
Which statements are correct?
I. The activation energy of a reaction is not affected by temperature.
II. A catalyst reduces the enthalpy change of a reaction.
III. Catalysts provide alternative reaction pathways.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Copper catalyses the reaction between zinc and dilute sulfuric acid.
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
Why does copper affect the reaction?
A. Decreases the activation energy
B. Increases the activation energy
C. Increases the enthalpy change
D. Decreases the enthalpy change
Markscheme
A
Examiners report
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
Which methods can be used to monitor the progress of this reaction?
\(\begin{array}{*{20}{l}} {{\text{I.}}}&{{\text{Change in colour of this reaction mixture}}} \\ {{\text{II.}}}&{{\text{Change in mass of this reaction mixture}}} \\ {{\text{III.}}}&{{\text{Change in volume of gas evolved}}} \end{array}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
Which statement about the kinetic theory is not correct?
A. The particles in ice vibrate about fixed points.
B. The particles in steam have more energy than the particles in ice.
C. All the particles in water have the same amount of energy at 298 K.
D. Evaporation of water occurs at all temperatures between 273 K and 373 K when the atmospheric pressure is 101 kPa.
Markscheme
C
Examiners report
There were two G2 comments on this question both of which stated that this was a difficult question at SL. The question certainly was challenging and was found to be the third hardest question on the paper. However, 49% of candidates did manage to get the correct answer, C.
Hydrochloric acid is reacted with large pieces of calcium carbonate, the reaction is then repeated using calcium carbonate powder. How does this change affect the activation energy and the collision frequency?
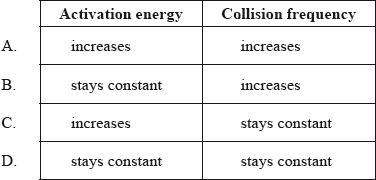
Markscheme
B
Examiners report
Why does the rate of a reaction increase when the temperature is increased?
I. The activation energy decreases.
II. There are more particles with energy equal to or greater than the activation energy.
III. The frequency of collisions between particles increases.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
Which graph best represents the relationship between the average kinetic energy of molecules of a gas and temperature in K?
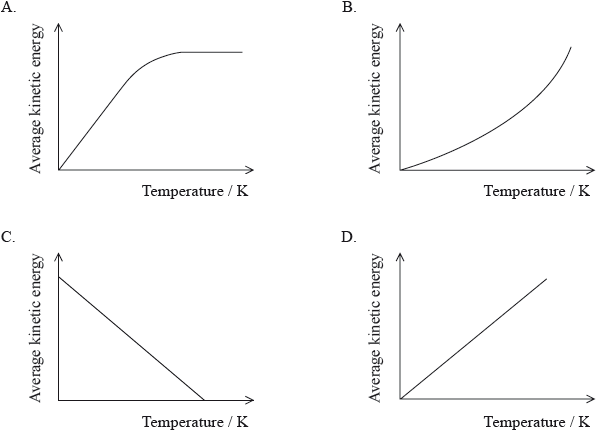
Markscheme
D
Examiners report
Some respondents mentioned in their G2 forms that the question was not appropriate as they could not find in the syllabus details where the specific relationship between temperature in K and average kinetic energy of molecules of gas is mentioned. In assessment statement 6.2.1 in the syllabus details it is clearly stated that “average kinetic energy is proportional to temperature in kelvins”. 48.33% of the candidates chose the correct answer D. The discrimination index for this question was 0.42 which is reasonably good.
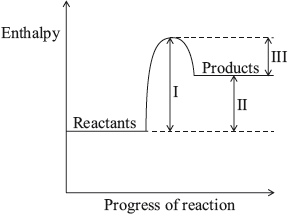
The diagram below shows the energy changes for a reaction with and without a catalyst. Which symbols represent the activation energy, \({E_{\text{a}}}\), and the enthalpy change, \(\Delta H\), for the reaction with a catalyst?
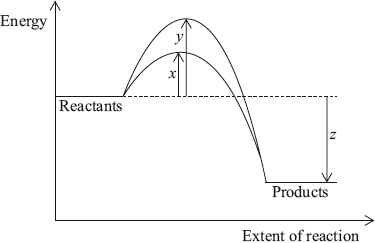
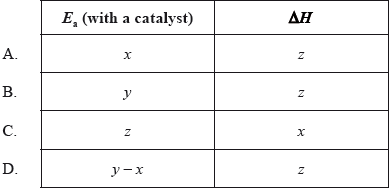
Markscheme
A
Examiners report
One respondent commented that it “is more difficult than it might be because of the use of algebra symbols”. This was the fourth easiest question with nearly 84% giving the correct answer.
Which changes increase the rate of the reaction below?
\[{{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{(g)}} + {\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \to {{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Cl (l)}} + {\text{HCl(g)}}\]
I. Increase of pressure
II. Increase of temperature
III. Removal of HCl(g)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
It was suggested by one respondent that increase of pressure might be ambiguous. In the guide, the effect of pressure on the rate of a reaction is mentioned in AS 6.2.4. 59% of candidates got this question correct.
Which factors can increase the rate of a chemical reaction?
I. Increasing the pressure in gaseous reactions
II. Increasing the temperature in gaseous reactions
III. Increasing the particle size of a solid in a reaction
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
There was a concern about the use of the word “can” in the question stem. The word was used to make the question about reactions in general. It does not seem to have troubled the candidates; it was the seventh easiest question, answered correctly by nearly 80% of the candidates.
Which statement best describes and explains the effect of a catalyst on the rate of a chemical reaction?
A. The rate increases because the frequency of collisions between particles increases.
B. The rate increases because more colliding particles have the energy needed to react.
C. The rate increases because the activation energy increases.
D. The rate increases because more molecules are present.
Markscheme
B
Examiners report
Which factors can affect the rate of reaction?
I. Particle size of solid reactant
II. Concentration of reacting solution
III. Pressure of reacting gas
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
Which factors can affect reaction rate?
I. The state of the reactants
II. The frequency of the collisions between particles
III. The average kinetic energy of the particles
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
The fact that the state of the reactants is inevitably linked to the frequency of collisions between particles appeared to cause a degree of confusion as to whether they were separate factors and as a result the question also proved a poor discriminator, with a discrimination index of 0.04. A decision was therefore taken to omit it from the total mark.
Which are appropriate units for the rate of a reaction?
A. \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{{\text{s}}^{ - 1}}\)
B. \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{s}}\)
C. \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\)
D. s
Markscheme
A
Examiners report
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
Which change does not increase the initial rate of reaction when CaCO3(s) is added to excess HCl(aq)?
A. Decrease in the size of the CaCO3(s) particles
B. Increase in the temperature of the reaction mixture
C. Increase in the concentration of HCl(aq), keeping the same volume
D. Increase in the volume of HCl(aq), keeping the same concentration
Markscheme
D
Examiners report
The following enthalpy level diagram shows the effect of the addition of a catalyst on a chemical reaction. What do m, n and o represent?
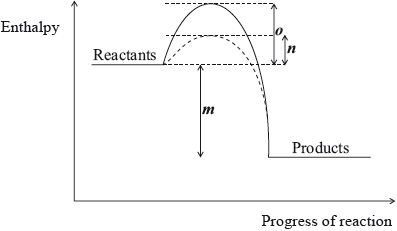

Markscheme
D
Examiners report
In which flask will the reaction between 2.0 g of magnesium carbonate and 25 cm3 1.0 mol dm–3 hydrochloric acid occur most rapidly?
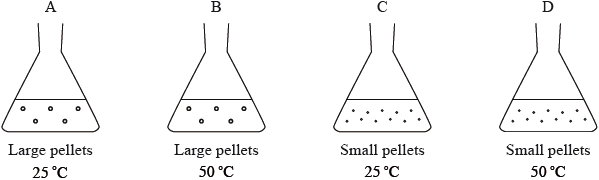
Markscheme
D
Examiners report
Although one respondent was concerned about the circles being confused with bubbles and the use of the word “pellet”, this turned out to be the easiest question on the paper. “Pellet” is a word that the examiners would expect candidates to understand.
A piece of zinc was added to aqueous nitric acid and the volume of hydrogen gas produced was measured every minute. The results are plotted on the graph below.
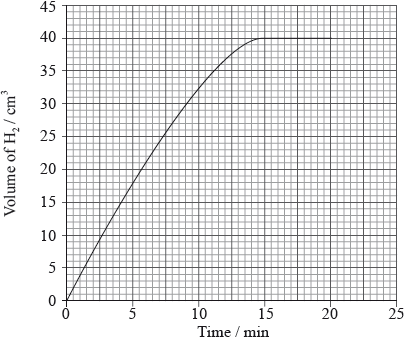
Which graph would you expect if the same mass of powdered zinc was added to nitric acid with the same concentration?
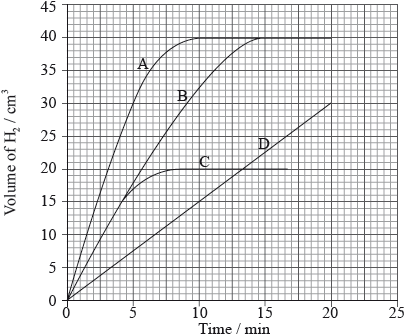
Markscheme
A
Examiners report
Nitrogen gas reacts with hydrogen gas according to the following equation.
\[{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\;\;\;\;\;\Delta H = - 92{\text{ kJ}}\]
Why is the rate of reaction slow at room temperature?
A. The activation energy of the forward reaction is high.
B. The activation energy of the forward reaction is low.
C. The equilibrium constant is very small.
D. The rate of the reverse reaction is greater than the rate of the forward reaction.
Markscheme
A
Examiners report
Which statement is true about using sulfuric acid as a catalyst in the following reaction?
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{–CO–C}}{{\text{H}}_{\text{3}}}{\text{(aq)}} + {{\text{I}}_{\text{2}}}{\text{(aq)}}\xrightarrow{{{{\text{H}}^ + }{\text{(aq)}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{–CO–C}}{{\text{H}}_{\text{2}}}{\text{–I(aq)}} + {\text{HI(aq)}}\]
I. The catalyst increases the rate of reaction.
II. The catalyst lowers the activation energy for the reaction.
III. The catalyst has been consumed at the end of the chemical reaction.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
Five respondents alluded to the fact that the three options were listed as I, II and II, instead of I, II and III. The question overall was well answered by candidates (Difficulty Index was 76%). Another respondent stated that statement II, the fact the catalyst lowers the activation energy for the reaction is in fact misleading, as a catalyst provides in fact an alternative reaction pathway with a lower activation energy requirement. This is a valid interpretation, but it was felt that the wording did not have an impact on student performance.
Consider the reaction between gaseous iodine and gaseous hydrogen.
\[\begin{array}{*{20}{l}} {{{\text{I}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \rightleftharpoons {\text{2HI(g)}}}&{\Delta {H^\Theta } = - 9{\text{ kJ}}} \end{array}\]
Why do some collisions between iodine and hydrogen not result in the formation of the product?
A. The \({{\text{I}}_{\text{2}}}\) and \({{\text{H}}_{\text{2}}}\) molecules do not have sufficient energy.
B. The system is in equilibrium.
C. The temperature of the system is too high.
D. The activation energy for this reaction is very low.
Markscheme
A
Examiners report
Which of the following can increase the rate of a chemical reaction?
I. Increasing the temperature
II. Adding a catalyst
III. Increasing the concentration of reactants
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
Which conditions must be met for a reaction to take place?
I. Reactants collide with sufficient energy.
II. Reactants collide with correct orientation.
III. Reactants must be in the same state.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
Equal masses of powdered calcium carbonate were added to separate solutions of hydrochloric acid. The calcium carbonate was in excess. The volume of carbon dioxide produced was measured at regular intervals. Which curves best represent the evolution of carbon dioxide against time for the acid solutions shown in the table below.
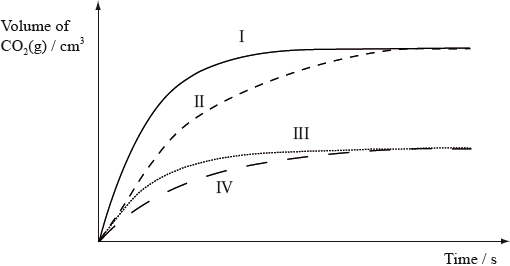
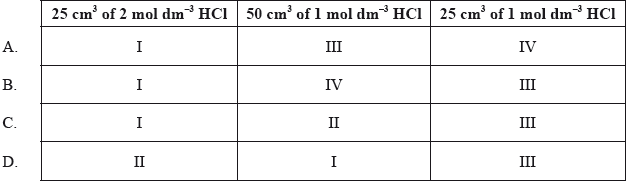
Markscheme
C
Examiners report
Opinion expressed through the G2 forms was divided with some commenting that this was a good question, whilst others felt it was too time consuming. Though a significant number of blank responses would seem to indicate that some candidates found the format of the question confusing, those that answered it performed quite well as indicated by a difficulty index of 44%. The question also proved to be quite a good discriminator, with a discrimination index of 0.33.
Which change increases the rate of a chemical reaction?
A. Increasing the size of solid reactant particles
B. Decreasing the concentration of aqueous reactants
C. Increasing the surface area of a solid reactant
D. Decreasing the pressure of gaseous reactants
Markscheme
C
Examiners report
Which is not affected by an increase in temperature?
A. Rate of reaction
B. Collision frequency
C. Collision geometry
D. % of molecules with \(E \ge {E_a}\)
Markscheme
C
Examiners report
Which change increases the rate of formation of hydrogen when zinc reacts with excess hydrochloric acid, assuming all other conditions remain the same?
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
A. Adding water to the hydrochloric acid
B. Decreasing the temperature
C. Increasing the volume of hydrochloric acid
D. Decreasing the size of the zinc particles while keeping the total mass of zinc the same
Markscheme
D
Examiners report
The potential energy profile for the reversible reaction, X + Y \( \rightleftharpoons \) Z is shown.
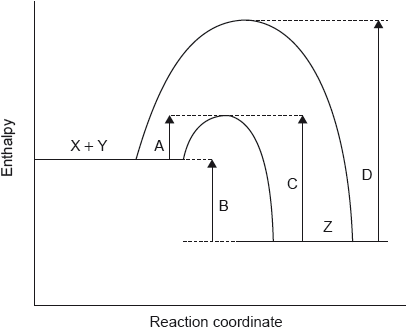
Which arrow represents the activation energy for the reverse reaction, Z → X + Y, with a catalyst?
Markscheme
C
Examiners report
Which piece of equipment could not be used in an experiment to measure the rate of this reaction?
\[{\text{C}}{{\text{H}}_3}{\text{COC}}{{\text{H}}_3}{\text{(aq)}} + {{\text{I}}_2}{\text{(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{COC}}{{\text{H}}_2}{\text{I (aq)}} + {{\text{H}}^ + }{\text{(aq)}} + {{\text{I}}^ - }{\text{(aq)}}\]
A. A colorimeter
B. A gas syringe
C. A stopwatch
D. A pH meter
Markscheme
B
Examiners report
\({\text{100 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of hydrochloric acid is added to 2.00 g of small pieces of calcium carbonate at 20 °C. The volume of carbon dioxide produced against time is plotted to give curve P.
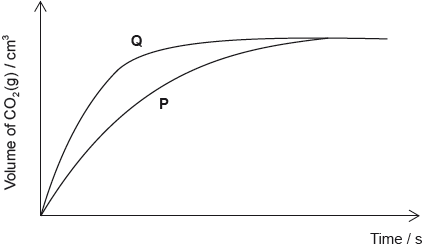
Which change will produce curve Q, given that calcium carbonate is always the limiting reagent?
A. Increasing the volume of the hydrochloric acid to \({\text{200 c}}{{\text{m}}^{\text{3}}}\)
B. Increasing the mass of calcium carbonate to 4.00 g
C. Increasing the concentration of the hydrochloric acid to \({\text{2.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\)
D. Replacing the 2.00 g of small pieces of calcium carbonate with 2.00 g of larger pieces of calcium carbonate
Markscheme
C
Examiners report
The diagram represents the Maxwell‒Boltzmann energy distribution curve of the reactants for a chemical reaction with different activation energies, \({E_{{\text{a1}}}}\) and \({E_{{\text{a2}}}}\).

What is the reason why the rate of the reaction with activation energy \({E_{{\text{a2}}}}\) is greater?
A. More frequent collisions between the particles occur.
B. More energetic collisions between the particles occur.
C. A catalyst has been added.
D. The temperature is higher.
Markscheme
C
Examiners report
The initial wording of the question was thought to be complicated, though it was clear that a catalyst had been added (only possible answer here).
Which unit could be used for the rate of a chemical reaction?
A. mol
B. \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\)
C. \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{{\text{s}}^{ - 1}}\)
D. \({\text{d}}{{\text{m}}^{\text{3}}}\)
Markscheme
C
Examiners report
Excess magnesium powder was added to a beaker containing hydrochloric acid, HCl (aq).
The mass of the beaker and its contents was recorded and plotted against time (line I).
Which change could give line II?
A. Doubling the mass of powdered Mg
B. Using the same mass of Mg ribbon
C. Increasing the temperature
D. Using the same volume of more concentrated HCl
Markscheme
D
Examiners report
For the reaction R → P, which letter represents the activation energy for the catalysed reverse reaction?
Markscheme
C
Examiners report
Which quantity can be changed by the use of a catalyst?
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
What is the best definition of rate of reaction?
A. The time it takes to use up all the reactants
B. The rate at which all the reactants are used up
C. The time it takes for one of the reactants to be used up
D. The increase in concentration of a product per unit time
Markscheme
D
Examiners report
A student added 0.20 g of calcium carbonate powder to \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid (an excess) and measured the volume of the gas that was evolved. The graph of the results is shown below.
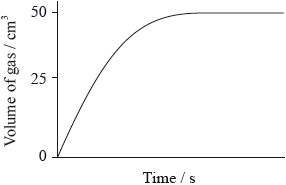
Which graph would be obtained if 0.20 g of calcium carbonate powder is added to \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.5 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid (an excess)?
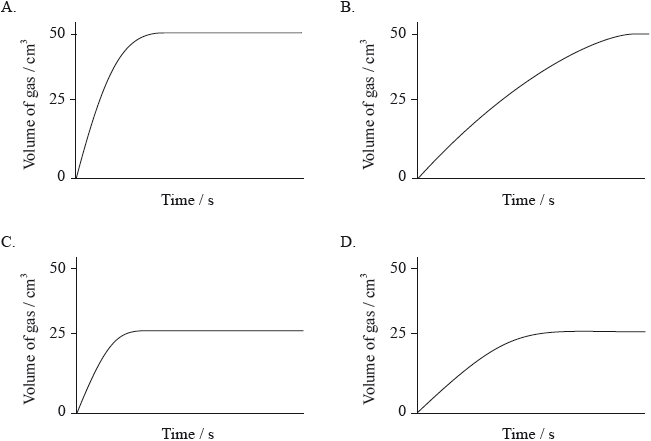
Markscheme
B
Examiners report
Which is a correct unit for expressing the rate of a reaction?
A. \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{{\text{s}}^{ - 1}}\)
B. \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{s}}\)
C. \({\text{mol}}\,{\text{s}}\)
D. \({\text{mo}}{{\text{l}}^{ - 1}}\,{\text{d}}{{\text{m}}^{\text{3}}}{{\text{s}}^{ - 1}}\)
Markscheme
A
Examiners report
Which variable is best to use when determining the rate of decomposition of hydrogen peroxide?
\[{\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(l)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\]
A. Volume of solution
B. Volume of gas
C. pH of solution
D. Conductivity of solution
Markscheme
B
Examiners report
Consider the following reaction between hydrogen peroxide, hydrogen ions and iodide ions.
\[{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{I}}^ - }{\text{(aq)}} \to {{\text{I}}_{\text{2}}}{\text{(aq)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
Which changes could be used to investigate the rate of this reaction?
I. Electrical conductivity
II. Mass of solution
III. Colour intensity
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Which statements explain the increase in the rate of a reaction when the temperature is increased?
I. More particles have energy greater than the activation energy.
II. The frequency of collisions increases.
III. The activation energy decreases.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
100 cm3 of 10% hydrogen peroxide solution decomposes at 298 K to form water and oxygen.
H2O2(aq) → H2O(l) + \(\frac{1}{2}\)O2(g)
The dotted line graph represents the volume of oxygen produced.
Which graph represents the decomposition of an equal volume of a 20% solution under the same conditions?
Markscheme
A
Examiners report
The diagram shows the energy profile for a catalysed and uncatalysed reaction.
Which represents the enthalpy change, ΔH, and the activation energy, Ea, for the catalysed reaction?
Markscheme
A
Examiners report
Graph 1 shows a plot of volume of CO2(g) against time for the reaction of CaCO3(s) with 1.00 moldm−3HCl (aq). The acid is the limiting reagent and entirely covers the lumps of CaCO3(s).
Which set of conditions is most likely to give the data plotted in graph 2 when the same mass of CaCO3(s) is reacted with the same volume of HCl(aq) at the same temperature?
Markscheme
C